내 암세포의 ‘먼 친척’ 찾아내 암 예방주사 만든다.
지난 4월 미 스탠퍼드 대학 연구팀이 암 예방주사를 만들어 쥐에 주사했다는 희소식을 전했다. 이후 암이 생기지 않고 예방됐다한다. 게다가 환자맞춤형이다. 이게 어떻게 가능할까?
암 예방으로 생명을 구한다면 그게 최우선이다.” 유방암 수술을 한 앤젤리나 졸리의 말이다.
Cancer vaccines are back in the headlines with research teams from Stanford University School of Medicine publishing breakthrough results of immunotherapy studies. Cancer research seems to be experiencing a tidal wave of immunotherapy treatment options; the excitement around the US Food and Drug Administration approval of the first CAR T-cell therapy and then a second one within months, is still afresh. The Stanford teams are taking this to a new level by pressing all the right buttons for a cancer vaccine.
This February, an interesting study led by researchers at the university has shown experimental vaccines as possible treatments for cancers in mice, and the university is starting human trials soon.
담배 근처에 가지도 않았는데 폐암이 생겼다. 억울하다.
Cancer vaccines are back in the headlines with research teams from Stanford University School of Medicine publishing breakthrough results of immunotherapy studies. Cancer research seems to be experiencing a tidal wave of immunotherapy treatment options; the excitement around the US Food and Drug Administration approval of the first CAR T-cell therapy and then a second one within months, is still afresh. The Stanford teams are taking this to a new level by pressing all the right buttons for a cancer vaccine.
This February, an interesting study led by researchers at the university has shown experimental vaccines as possible treatments for cancers in mice, and the university is starting human trials soon.
📷 : 암세포(중앙)를 둘러싼 면역T세포들.
예방주사는 이런 특정 면역세포들을 미리 준비케 한다. 쥐를 대상으로 한 암 예방주사 실험이 성공했다
담배 근처에 가지도 않았는데 폐암이 생겼다. 억울하다.
하지만 2017년 미 존스홉킨스대학 연구에 의하면 암 67%가 무작위로 생긴다. 운이 없으면 걸린다. 결국 믿을 놈은 하나다. 생기는 족족 잡아 줄 내 면역뿐이다. 이게 약해졌다면 불안하다.
쥐 역분화쥐 역분화 줄기세포 죽여서 주사
예방주사는 해당 균을 미리 주사해서 이를 기억하는 면역세포를 만들어 놓는다. 살아 있으면 위험하니 죽인 균이나 껍질 성분(항원)만 주사한다. 암세포도 직접 주사하면 위험하니 죽이거나 껍질 성분(항원)만 주사하면 된다. 문제는 걸리기 전에는 내 암세포를 미리 구할 수가 없다는 거다. 내 암세포를 가장 닮은 놈을 찾아내면 된다.
왜 다른 예방주사(백신)처럼 한번 맞으면 평생 가는 ‘암 예방주사’는 없을까?
암은 종류 따라, 환자 따라 각각 다르다. 또 쉽게 변해 버린다. 암 예방주사가 어려운 이유다.
Stimulating immune system against solid tumors
The study injected minute amounts of two immune-stimulating agents directly into solid tumours in mice and found that this could eliminate all traces of cancer in the animals, including distant, untreated metastases.
“When we use these two agents together, we see the elimination of tumors all over the body,” said Ronald Levy, MD, professor of oncology and senior author of the study, in the Stanford press release.
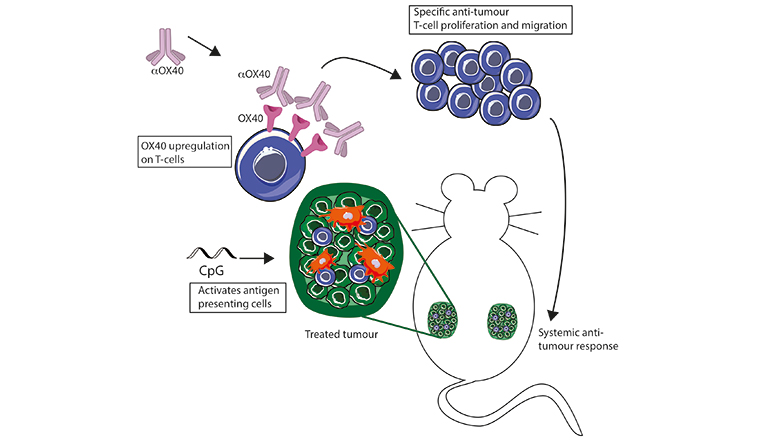
The cancer environment displays a strange kind of relationship with the immune system. Immune cells like T-cells recognize the abnormal proteins present on the surface of cancer cells and infiltrate the cells to attack the tumor. However, as the tumor grows, it devises ways to suppress this activity of the T-cells.
The new method reactivates T-cells and harnesses a much stronger immune response against the tumor. It uses a combination of two molecules; the first is a short piece of DNA or the CpG oligonucleotide causing the T-cells to increase expression of receptor molecules, OX40, and the other is an antibody that binds with the OX40, which activates the T-cells to amplify the anti-cancer response. Dr Idit Sagiv-Barfi, the lead author of the study, explains, “We use a combination of two reagents. The first, CpG, is a small DNA sequence that has properties of microbial/bacterial DNA unlike human DNA. When it is injected into the tumor, immune cells in the tumor sense it as foreign DNA and begin a signaling cascade eventually leading to a local immune response. T-cells are among the cells that are activated, as a result of this activation they increase the expression of a protein known as OX40 on their surface. The second reagent in our combinational therapy is an agonistic antibody targeting OX40. This antibody binds its target, and causes activation of a subset of T-cells known as T effector cells and reduces the activity of a different subset of T-cells known as T regulatory cells (T regs). T regs are suppressors of T effector cell. So, by combining both reagents we are activating the activators and suppressing the suppressors, and this way we switch the balance from an immune-suppressive to an immune-promoting environment.”
One of the most interesting aspects of the study was that some of the tumour-specific, activated T-cells left the original tumour to find and destroy other identical tumours throughout the body, thereby displaying an overall body response.
“The biggest risk of cancer is not necessarily the primary tumour, but the metastasis. For this reason, in our experiments, we implanted tumours at two different sites of the mouse’s abdomen. We treated only one of those tumours with our combination (CpG and an antibody) and monitored the tumour development in both the treated and the non-treated tumour. We found that the combination of CpG and an antibody against OX40 was sufficient to regress the treated tumour and then, a few days later, the non-treated tumour also completely regressed. We waited three months after the mice were completely tumour free and then re-challenged those mice again with the same tumour cells, and none of them developed tumours. They were completely protected against the tumour challenge, meaning that they now had immune-memory against the tumour,” clarifies Sagiv-Barfi.
The vaccine was tested on 90 different mice, and the results were better than expected, with all traces of cancer effectively eliminated in 87 of the mice, including distant untreated metastases.
This new vaccine is entering human trials. The plan is to enroll 15 patients with low grade lymphoma. Sagiv-Barfi says, “The reason we chose lymphoma for the first trial is that we have conducted trials with CpG in lymphoma, it is the cancer of the immune system and easier to monitor response in an indolent subset of patients.”
One of the goals of the planned clinical trial is to assess the safety of the combination. Both reagents have already been tested in clinical trials as single agents and were well tolerated. The trial will use one in hundredth of the dose that was used for systemic administration of anti-OX40 since they will be injecting directly into the tumour. This targeted approach may also aid in avoiding harsh side effects.
“We plan to open a new trial to solid tumours if this trial is safe and successful”, adds Sagiv-Barfi.
What is so different about this solution? The study published in the Science Translational Medicine bypassed the need to identify tumour-specific immune targets, or customisation of a patient’s immune cells or activation of the entire immune system. Unlike in methods involving adoptive cell transfer, where there is an actual transfer of cells to the patient that originated from the tumour or engineered to the CAR T-cells and expanded outside the body, the new vaccine study led by Levy and Sagiv-Barfi stimulated T-cells within the patient’s body to cause an immune response against the tumour without the need to transfer any cells.
“This is not a personalized therapy, so hopefully the price tag will not be as high,” says Sagiv-Barfi. If the trial is successful, the treatment could be useful in many types of tumors. The researchers are hopeful that this can be used in future cancer therapy.
This article was first published in The Week magazine dated July 29, 2018 under the title, Taking it to the next level.
Stimulating immune system against solid tumors
The study injected minute amounts of two immune-stimulating agents directly into solid tumours in mice and found that this could eliminate all traces of cancer in the animals, including distant, untreated metastases.
“When we use these two agents together, we see the elimination of tumors all over the body,” said Ronald Levy, MD, professor of oncology and senior author of the study, in the Stanford press release.

The cancer environment displays a strange kind of relationship with the immune system. Immune cells like T-cells recognize the abnormal proteins present on the surface of cancer cells and infiltrate the cells to attack the tumor. However, as the tumor grows, it devises ways to suppress this activity of the T-cells.
The new method reactivates T-cells and harnesses a much stronger immune response against the tumor. It uses a combination of two molecules; the first is a short piece of DNA or the CpG oligonucleotide causing the T-cells to increase expression of receptor molecules, OX40, and the other is an antibody that binds with the OX40, which activates the T-cells to amplify the anti-cancer response. Dr Idit Sagiv-Barfi, the lead author of the study, explains, “We use a combination of two reagents. The first, CpG, is a small DNA sequence that has properties of microbial/bacterial DNA unlike human DNA. When it is injected into the tumor, immune cells in the tumor sense it as foreign DNA and begin a signaling cascade eventually leading to a local immune response. T-cells are among the cells that are activated, as a result of this activation they increase the expression of a protein known as OX40 on their surface. The second reagent in our combinational therapy is an agonistic antibody targeting OX40. This antibody binds its target, and causes activation of a subset of T-cells known as T effector cells and reduces the activity of a different subset of T-cells known as T regulatory cells (T regs). T regs are suppressors of T effector cell. So, by combining both reagents we are activating the activators and suppressing the suppressors, and this way we switch the balance from an immune-suppressive to an immune-promoting environment.”
One of the most interesting aspects of the study was that some of the tumour-specific, activated T-cells left the original tumour to find and destroy other identical tumours throughout the body, thereby displaying an overall body response.
“The biggest risk of cancer is not necessarily the primary tumour, but the metastasis. For this reason, in our experiments, we implanted tumours at two different sites of the mouse’s abdomen. We treated only one of those tumours with our combination (CpG and an antibody) and monitored the tumour development in both the treated and the non-treated tumour. We found that the combination of CpG and an antibody against OX40 was sufficient to regress the treated tumour and then, a few days later, the non-treated tumour also completely regressed. We waited three months after the mice were completely tumour free and then re-challenged those mice again with the same tumour cells, and none of them developed tumours. They were completely protected against the tumour challenge, meaning that they now had immune-memory against the tumour,” clarifies Sagiv-Barfi.
The vaccine was tested on 90 different mice, and the results were better than expected, with all traces of cancer effectively eliminated in 87 of the mice, including distant untreated metastases.
This new vaccine is entering human trials. The plan is to enroll 15 patients with low grade lymphoma. Sagiv-Barfi says, “The reason we chose lymphoma for the first trial is that we have conducted trials with CpG in lymphoma, it is the cancer of the immune system and easier to monitor response in an indolent subset of patients.”
One of the goals of the planned clinical trial is to assess the safety of the combination. Both reagents have already been tested in clinical trials as single agents and were well tolerated. The trial will use one in hundredth of the dose that was used for systemic administration of anti-OX40 since they will be injecting directly into the tumour. This targeted approach may also aid in avoiding harsh side effects.
“We plan to open a new trial to solid tumours if this trial is safe and successful”, adds Sagiv-Barfi.
What is so different about this solution? The study published in the Science Translational Medicine bypassed the need to identify tumour-specific immune targets, or customisation of a patient’s immune cells or activation of the entire immune system. Unlike in methods involving adoptive cell transfer, where there is an actual transfer of cells to the patient that originated from the tumour or engineered to the CAR T-cells and expanded outside the body, the new vaccine study led by Levy and Sagiv-Barfi stimulated T-cells within the patient’s body to cause an immune response against the tumour without the need to transfer any cells.
“This is not a personalized therapy, so hopefully the price tag will not be as high,” says Sagiv-Barfi. If the trial is successful, the treatment could be useful in many types of tumors. The researchers are hopeful that this can be used in future cancer therapy.
This article was first published in The Week magazine dated July 29, 2018 under the title, Taking it to the next level.
쥐 역분화쥐 역분화 줄기세포 죽여서 주사
예방주사는 해당 균을 미리 주사해서 이를 기억하는 면역세포를 만들어 놓는다. 살아 있으면 위험하니 죽인 균이나 껍질 성분(항원)만 주사한다. 암세포도 직접 주사하면 위험하니 죽이거나 껍질 성분(항원)만 주사하면 된다. 문제는 걸리기 전에는 내 암세포를 미리 구할 수가 없다는 거다. 내 암세포를 가장 닮은 놈을 찾아내면 된다.
유방·피부암, 중피종 등 예방 가능
스탠퍼드 의대 연구진은 쥐 피부세포를 떼어내 줄기세포(역분화)로 만들었다.
스탠퍼드 의대 연구진은 쥐 피부세포를 떼어내 줄기세포(역분화)로 만들었다.
이놈을 죽여 암 예방주사를 만들었다. 이를 쥐에게 주사 후 유방암 세포 40만 개를 주입했다. 예방주사를 맞지 않은 쥐는 주입한 유방암세포가 자라 암 덩어리가 생겼다. 반면 예방주사를 맞은 쥐 70%는 암이 생기지 않았다. 유방암뿐만 아니라 중피종, 피부암 등도 모두 같은 예방 효과를 냈다. 왜 암세포도 아닌 역분화 줄기세포가 면역세포들에 암세포로 인식되었을까. 그 답은 산모 입덧 속에 있다.
산모는 임신 초기에 입덧을 한다. 나쁜 음식을 예방한다는 해석도 있다.
산모는 임신 초기에 입덧을 한다. 나쁜 음식을 예방한다는 해석도 있다.
입덧은 호르몬(hCG·인간 융모성 생식선 자극 호르몬)이 솟구치는 시기와 일치한다. 이 물질은 놀랍게도 암세포에서도 나온다. 왜 이 시기에 산모는 암세포와 유사한 호르몬을 내놓을까. 그 답은 산모 탯줄 속에 있다.
탯줄은 예로부터 보관 항아리를 따로 만들 만큼 귀히 여겼다. 지금은 그곳에 줄기세포가 많다 해서 냉동보관도 한다. 정작 중요한 건 탯줄 만드는 ‘착상’ 과정이다. 착상은 해안절벽에 배를 고정시키는 군사작전을 방불케 한다. 수정란이 분열해서 배아가 된다. 이 배아가 자궁벽에 달라붙는다. 배아껍질세포(영양막 세포)가 침투조다. 벽에 작은 구멍을 낸다. 한 발을 디밀고 틈새로 몸을 밀어 넣는다. 조금씩 더 비집고 들어간다. 수를 불린다. 이어 근처 혈관 벽을 허물고 새로운 혈관을 만들어 끌고 온다. 혈관을 엮어 탯줄을 완성한다. 임신 성공이다.
배아세포 : 암처럼 침투·증식·전이
수훈은 배아껍질세포다.
탯줄은 예로부터 보관 항아리를 따로 만들 만큼 귀히 여겼다. 지금은 그곳에 줄기세포가 많다 해서 냉동보관도 한다. 정작 중요한 건 탯줄 만드는 ‘착상’ 과정이다. 착상은 해안절벽에 배를 고정시키는 군사작전을 방불케 한다. 수정란이 분열해서 배아가 된다. 이 배아가 자궁벽에 달라붙는다. 배아껍질세포(영양막 세포)가 침투조다. 벽에 작은 구멍을 낸다. 한 발을 디밀고 틈새로 몸을 밀어 넣는다. 조금씩 더 비집고 들어간다. 수를 불린다. 이어 근처 혈관 벽을 허물고 새로운 혈관을 만들어 끌고 온다. 혈관을 엮어 탯줄을 완성한다. 임신 성공이다.
배아세포 : 암처럼 침투·증식·전이
수훈은 배아껍질세포다.
이놈들은 자궁벽에 달라붙고, 침입하고, 옮겨가고, 수를 불리고, 혈관을 만들었다. 어디선가 많이 들어본 침투-증식-전이 작전이다. 그렇다. 바로 암세포가 하는 일이다. 산모 속 배아는 행동거지가 암세포를 빼닮았다. 실제로 3개월 된 임산부와 소화기 암환자의 혈액 면역성분은 80% 유사하다. 유방암 5개, 대장암 11개. 난소암 10개, 폐암 5개 성분이 산모배아성분과 정확히 같다. 그럼 배아세포로 암 예방주사를 만들면 어떨까.
배아 사용은 현실적으로 어렵다.
배아 사용은 현실적으로 어렵다.
생명체다. 윤리문제가 불거진다. 배아와 가장 닮은 건 원시상태세포, 즉 줄기세포다. 그중에서도 역분화 줄기세포가 최적이다. 피부세포를 ‘리셋’시켜 쉽게 만든다. 윤리문제가 없다. 게다가 자기 세포로 만드니 자기 암세포를 닮았다. 개인맞춤형 암 예방주사로는 최고다.
그동안 암 예방주사가 실패했던 이유는 암세포가 쉽게 변하기 때문이다.
그동안 암 예방주사가 실패했던 이유는 암세포가 쉽게 변하기 때문이다.
즉 암세포 표면 한 개 표적(항원)만을 목표로 예방주사를 만들면 암세포는 그 표적물질(항원)을 더는 안 만들게 변한다. 따라서 최대한 많은 표적을 대상으로 해야 한다. 더 좋기는 개인별 고유한 표적도 포함해야 한다. 결국 최고 예방주사는 개인 암세포 ‘통째’다. 암세포는 암에 걸리기 전에는 구할 수 없다. 따라서 암세포 특성을 가장 닮은 배아 껍질세포, 그놈을 가장 닮은 원시상태 역분화 줄기세포를 죽여서 미리 주사하는 거다.
착상과정은 또 다른 아이디어를 준다.
착상과정은 또 다른 아이디어를 준다.
즉 정상적인 배아껍질세포가 어떻게 암세포로 변하는가, 그리고 착상임무가 끝나면 어떻게 정상세포로 되는 가이다. 이 단계를 잘 들여다보면 중요한 암 치료 단서를 찾을 수 있다.
이번 연구가 사람에게도 적용된다면 암 걱정은 하지 않아도 될까.
이번 연구가 사람에게도 적용된다면 암 걱정은 하지 않아도 될까.
쥐 면역은 사람과 다르다. 하지만 암 표적물질 종류가 쥐나 인간 모두 비슷했다. 게다가 모든 포유류에서 배아껍질세포가 암처럼 침투해서 착상한다. 쥐도, 개도, 말도, 사람도 모두 유방암, 대장암, 폐암에 걸린다. 이번 연구가 사람에게도 같은 효과를 낼 것이라고 기대하는 이유다.
📚 Reference
https://med.stanford.edu/news/all-news/2018/01/cancer-vaccine-eliminates-tumors-in-mice.html
http://trialx.com/curetalk/2018/07/26/human-trials-for-cancer-vaccine-stanford-taking-it-to-the-next-level/
https://med.stanford.edu/news/all-news/2018/01/cancer-vaccine-eliminates-tumors-in-mice.html
http://trialx.com/curetalk/2018/07/26/human-trials-for-cancer-vaccine-stanford-taking-it-to-the-next-level/
https://news.joins.com/article/22932710


Comments